China’s First Gene-Editing Therapy for Beta-Thalassemia Receives FDA RMAT Designation
ART001, a gene-editing therapy developed by Accuredit Therapeutics, has become the first gene-editing treatment from China to receive the Regenerative Medicine Advanced Therapy (RMAT) designation from the U.S. Food and Drug Administration (FDA).
The therapy is designed to treat beta-thalassemia, a genetic blood disorder in which the body fails to produce sufficient beta-globin chains, leading to chronic anemia and a lifelong dependency on blood transfusions.
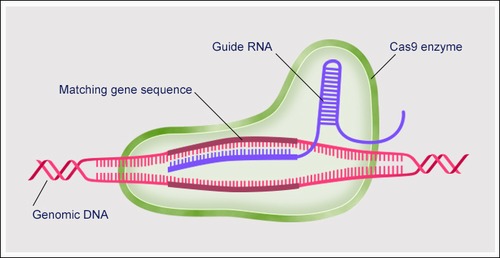
ART001 employs an ex vivo gene-editing approach by harvesting the patient's hematopoietic stem cells, correcting the faulty gene using technologies like CRISPR/Cas9, and reinfusing the edited cells into the body. These modified cells are capable of producing healthy blood components.
The RMAT designation is part of the FDA’s expedited pathways for promising regenerative therapies that address serious conditions and show early clinical effectiveness. It allows for more frequent interaction with the FDA, flexible trial designs, and potential for accelerated approval.
Dr. Zhao Jinxin, Chief Scientific Officer at Accuredit, described this milestone as a major advancement for China’s biotech sector and a significant step toward greater global participation in gene therapy innovation.
.png)

_1.png)

comment